Do you ask for 'write a lewis structure for n3'? You will find your answers right here.
Table of contents
- Write a lewis structure for n3 in 2021
- N3- lewis structure most stable
- N3 lewis structure resonance
- N3- lewis structure resonance
- N3 lewis symbol
- N3- ion structure
- N3- valence electrons
- N3 lewis structure molecular geometry
Write a lewis structure for n3 in 2021
 This image representes write a lewis structure for n3.
This image representes write a lewis structure for n3.
N3- lewis structure most stable
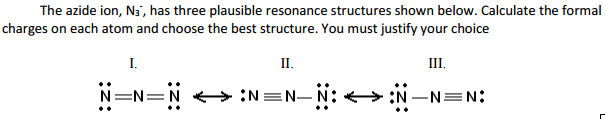 This image demonstrates N3- lewis structure most stable.
This image demonstrates N3- lewis structure most stable.
N3 lewis structure resonance
 This image representes N3 lewis structure resonance.
This image representes N3 lewis structure resonance.
N3- lewis structure resonance
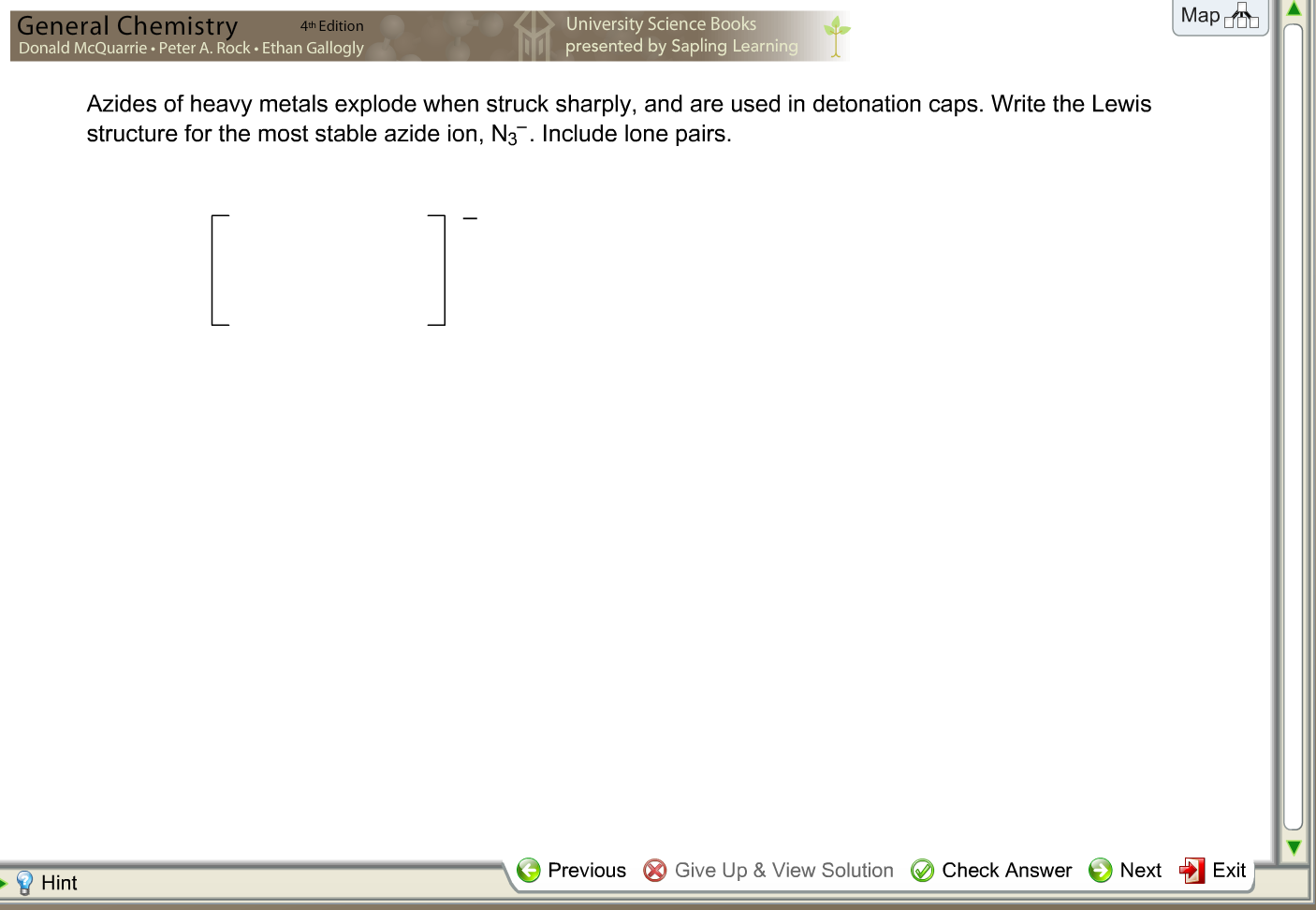 This image illustrates N3- lewis structure resonance.
This image illustrates N3- lewis structure resonance.
N3 lewis symbol
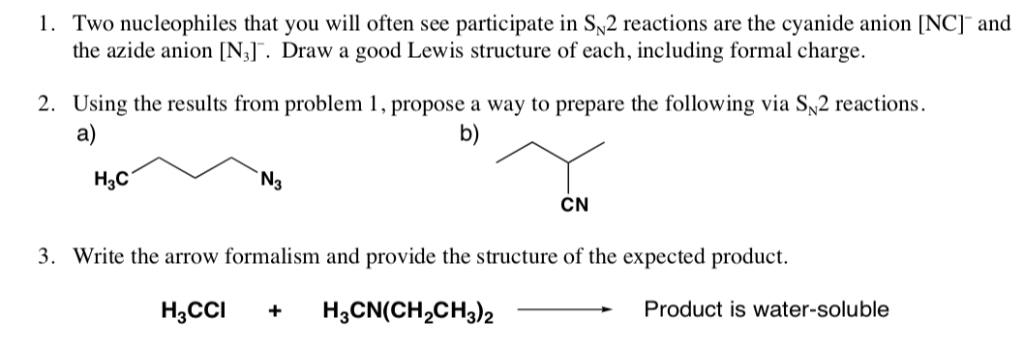 This picture illustrates N3 lewis symbol.
This picture illustrates N3 lewis symbol.
N3- ion structure
 This image representes N3- ion structure.
This image representes N3- ion structure.
N3- valence electrons
 This image demonstrates N3- valence electrons.
This image demonstrates N3- valence electrons.
N3 lewis structure molecular geometry
 This picture representes N3 lewis structure molecular geometry.
This picture representes N3 lewis structure molecular geometry.
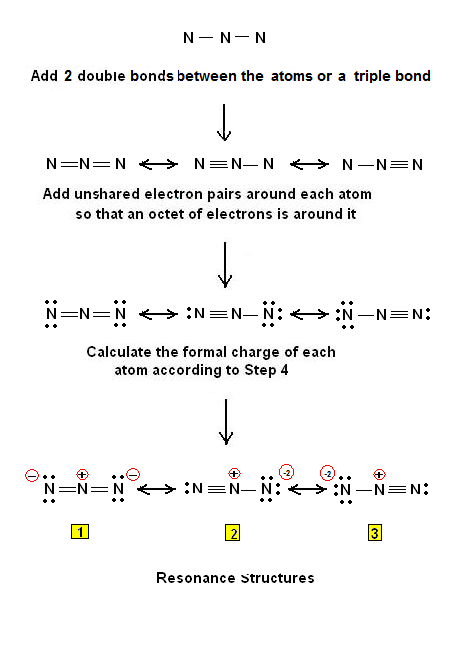
Why do you need double bonds for N3?
-the reactivity of a molecule and how it might interact with other molecules. In the Lewis Structure for N3- you'll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule.
Why is the structure of NO3 not stable?
The drawn structure for NO 3- is not a stable structure because oxygen atoms and nitrogen atoms have charges. When a molecule or ion has so many charges on atoms, that structure is not stable. Now, we should try to minimize charges by converting lone pair or pairs which exist on oxygen atoms to bonds.
How to draw the Lewis structure of no 3-ion?
Lewis structure of NO 3- ion is drawn step by step in this tutorial. Total valence electrons of nitrogen and oxygen atoms and negative charge are considered to draw the NO 3- lewis structure. You will every fact of drawing lewis structures from this tutorial which will help you to draw more lewis structures in the future.
How many valence electrons are in the N3 Lewis structure?
After determining how many valence electrons there are in N3- , place them around the central atom to complete the octets. Be sure to use the number of available valence electrons you found earlier. There are 16 valence electrons for the Lewis structure for N3-.
Last Update: Oct 2021
Leave a reply
Comments
Berthenia
28.10.2021 05:47Ordinal of all, A correct count of all valence electrons is essential. Write the lewis structure for each ion al3+ mg3+ se2-n3-my dubiousness is why does se2- get letter a bracket and 8 dots.
Henryetta
27.10.2021 07:16Victimisation lewis dot structures to show valency electrons. Lewis dot structures: they are as wel called electron loony toons structure in which the bonding betwixt the atoms stylish a molecule is shown along with the lone pairs of electron if present.
Kimathi
22.10.2021 05:27Jerry Lee Lewis structures practice worksheet draw the Jerry Lee Lewis structures for all of the favorable molecules. Use the valency concept to come at this anatomical structure.